[Financial News] June 23, 2018

Soonchunhyang University Bucheon Hospital begins full-scale patient recruitment.
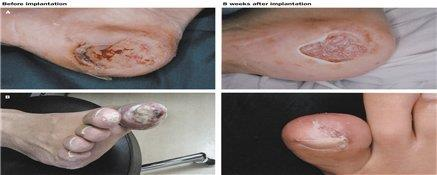
A path has been opened to treat diabetic severe limb ischemia using autologous bone marrow stem cells.
Miracell, a stem cell company, announced on the 23rd that its BMAC (Bio-Mechanical Axon Modulator) is effective in treating patients with diabetic severe limb ischemia. Autologous bone marrow stem cell therapy has been selected as a limited medical technology for the treatment of diabetic severe limb ischemia, a devastating condition that sometimes requires limb amputation. A support program has begun in earnest to establish clinical evidence for promising medical technologies in the research phase, where safety has been confirmed but clinical efficacy has been challenging to demonstrate. The limited medical technology assessment system allows patients with technologies that are not currently being introduced to Korean hospitals due to insufficient research on their therapeutic efficacy to receive treatment at hospitals and physicians designated by the Minister of Health and Welfare, provided that such technologies are available only when there are no other treatments available or when rapid adoption is required for patients with rare diseases.
We heard about the treatment outlook from Dr. Eun-soo Park, head of the department at Soonchunhyang University Bucheon Hospital, who is in charge of ‘Autologous bone marrow stem cell therapy for diabetic severe lower extremity ischemia’ and is currently recruiting patients.

―What is autologous bone marrow stem cell therapy for diabetic critical limb ischemia?
▲The number of patients with diabetes is rapidly increasing, currently reaching approximately 10% of the general population. Of these, 10-20% develop foot ulcers. These foot ulcers account for approximately 25% of all diabetes-related hospitalizations, and a significant number of these patients require limb amputation.
Peripheral vascular disease is the most important factor in the development of diabetic foot ulcers, and the average treatment period for diabetic critical limb ischemia is over six months. Conventional treatments, such as transplantation or intervention, are significantly limited.
Therefore, given the positive clinical trial reports on novel stem cell-based treatments, this clinical study aims to demonstrate the efficacy of autologous stem cell therapy for diabetic critical limb ischemia and establish a treatment method.
What is the purpose of autologous bone marrow stem cell therapy for diabetic critical limb ischemia?
▲This study investigated the clinical efficacy of autologous stem cells in improving diabetic critical limb ischemia. The aim was to apply autologous bone marrow-derived cells to patients with diabetic critical limb ischemia to induce angiogenesis, delay foot amputation, heal skin ulcers, and improve functional outcomes.
The purpose of this study was to evaluate the efficacy of autologous bone marrow stem cells in improving critical limb ischemia and to determine the potential complications or side effects.
What is “autologous bone marrow stem cell therapy for diabetic critical limb ischemia”?
▲This limited medical technology involves harvesting bone marrow, concentrating it, and then injecting it intramuscularly into the targeted area for patients with diabetic critical limb ischemia who are refractory to or unresponsive to conventional treatments.
By promoting angiogenesis and healing skin ulcers, the goal is to ultimately prevent foot amputations in patients suffering from diabetic critical limb ischemia.
―Eligibility for study participation:
① Age (male or female, 19 to 80 years of age).
② Patients diagnosed with diabetes and receiving oral medication or insulin therapy, or newly diagnosed with diabetes.
③ Patients who are unable or have failed bypass surgery.
④ Patients diagnosed with severe lower extremity ischemia (Rutherford classification 4-5) and experiencing symptoms despite conservative treatment. Symptoms include resting pain, non-healing wounds or gangrene of the belos metatarsals, an ankle-brachial index (ABI) < 0.7, or an ABI ≥ 0.7, but with toes that do not have a toe-brachial index (TBI) or arterial waveforms measured on plethysmography.
⑤ Participants must be willing and able to consent to the study, sign the consent form, and comply with the study requirements.
pompom@fnnews.com Jeong Myeong-jin, Medical Reporter
